
#CHROMIUM ATOMIC NUMBER SKIN#
When it is a compound in leather products, it can cause allergic reactions, such as skin rash. People who smoke tobacco also have a higher chance of exposure to chromium.Ĭhromium(VI) is known to cause various health effects. But the uptake of too much chromium(III) can cause health effects as well, for instance skin rashes.Ĭhromium(VI) is a danger to human health, mainly for people who work in the steel and textile industry. When food in stores in steel tanks or cans chromium concentrations may rise.Ĭhromium(III) is an essential nutrient for humans and shortages may cause heart conditions, disruptions of metabolisms and diabetes. Various ways of food preparation and storage may alter the chromium contents of food. For most people eating food that contains chromium(III) is the main route of chromium uptake, as chromium(III) occurs naturally in many vegetables, fruits, meats, yeasts and grains. In drinking water the level of chromium is usually low as well, but contaminated well water may contain the dangerous chromium(IV) hexavalent chromium. The level of chromium in air and water is generally low. People can be exposed to chromium through breathing, eating or drinking and through skin contact with chromium or chromium compounds. Reserves are hestimated to be of the order of 1 billion tonnes with unexploited deposits in Greenland, Canada e USA. A total of 14 million tonnes of chromite ore is extracted.

Chromium ores are mined today in South Africa, Zimbabwe, Finland, India, Kazakihstan and the Philippines. Chromium (IV) oxide (CrO 2) is used to manufacture magnetic tape.Ĭhromium is mined as chromite (FeCr 2O 4) ore.
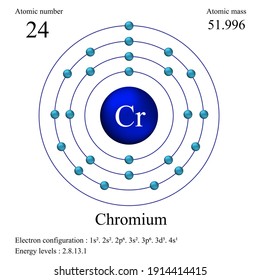

Chromium is used in metallurgy to impart corrosion resistance and a shiny finish as dyes and paints, its salts colour glass an emerald green and it is used to produce synthetic rubies as a catalyst in dyeing and in the tanning of leather to make molds for the firing of bricks. Chromium plating was once widely used to give steel a polished silvery mirror coating. ApplicationsĬhromium main uses are in alloys such as stainless steel, in chrome plating and in metal ceramics. Chromium is unstable in oxygen, it immediately produces a thin oxide layer that is impermeable to oxygen and protects the metal below. It does not tarnish in air, when heated it borns and forms the green chromic oxide. Its colour is silver-gray and it can be highly polished. Separation and Concentration Purification RequestĬhromium - Cr Chemical properties of chromium - Health effects of chromium - Environmental effects of chromiumĬhromium is a lustrous, brittle, hard metal.Plant Inspection & Process Optimalisation.The oxidation state of any chemically bonded carbon may be assigned by adding -1 for each bond to more electropositive atom (H, Na, Ca, B) and +1 for each bond to more electronegative atom (O, Cl, N, P), and 0 for each carbon atom bonded directly to the carbon of interest.The algebraic sum of the oxidation states in an ion is equal to the charge on the ion.Īssigning oxidation numbers to organic compounds.The algebraic sum of the oxidation numbers of elements in a compound is zero.Hydrogen has an oxidation number of +1 when combined with non-metals, but it has an oxidation number of -1 when combined with metals.Oxygen almost always has an oxidation number of -2, except in peroxides (H 2O 2) where it is -1 and in compounds with fluorine (OF 2) where it is +2.The alkaline earth metals (group II) are always assigned an oxidation number of +2.The alkali metals (group I) always have an oxidation number of +1.Fluorine in compounds is always assigned an oxidation number of -1.The oxidation number of a monatomic ion equals the charge of the ion.
#CHROMIUM ATOMIC NUMBER FREE#

Unlike radicals in organic molecules, R cannot be hydrogen. Organic compounds can be written in such a way that anything that doesn't change before the first C-C bond is replaced with the abbreviation R (Figure 1c). When dealing with organic compounds and formulas with multiple atoms of the same element, it's easier to work with molecular formulas and average oxidation numbers (Figure 1d). Notice that changing the CH 3 group with R does not change the oxidation number of the central atom. R is an abbreviation for any group in which a carbon atom is attached to the rest of the molecule by a C-C bond. Different ways of displaying oxidation numbers of ethanol and acetic acid.


 0 kommentar(er)
0 kommentar(er)
